|
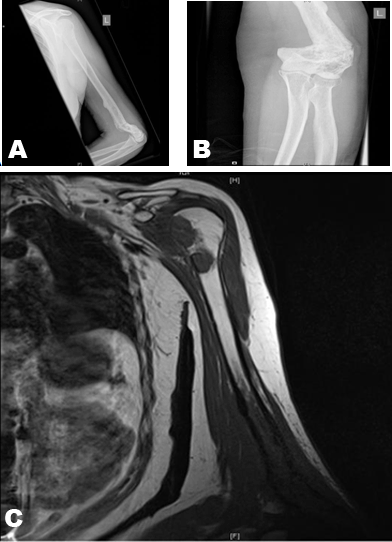
Metastatic tumors around the elbow are rare [4]. Usually, patients present with either pain or a pathological fracture. The surgical management of pathological fractures around the elbow is challenging, due to the uncommon nature of the condition and the lack of guidance in literature. The aim of treatment is to achieve pain relief and to restore maximum motion and stability, thus providing a functional upper limb.
With advances in imaging, staging and perioperative oncological management, life expectancy has increased and the debate as to whether salvage or amputate the limb has shifted to the former. Nowadays, reconstruction of the upper limb has become the standard treatment for patients with bone tumors. Compared to the other options of elbow reconstruction after bone resection, prosthetic replacement is the option of choice, in that arthrodesis is poorly tolerated and technically demanding, especially after tumor resection, osteoarticular allografts have a high complication rate and excision arthroplasty is rarely an option, due to the large bone gap [5]. For pathological fractures, open reduction and internal fixation is always a valid option, but it has a high nonunion rate [6]. Athwal et al., reported 20 patients after a Coonard Morey elbow replacement, undertaken for tumors of the distal ulnar. Although 18 patients also had perioperative radiotherapy/chemotherapy, the infection rate was 0%, 45% of patients experienced ulnar or radial nerve complication after surgery and local recurrence was 25%. These results demonstrated that a total elbow replacement gives good pain relief, preserves function and the oncological and non-oncological complications are comparable to other reconstruction techniques. These results demonstrated that within this clinical scenario, total elbow arthroplasty gives good pain relief.
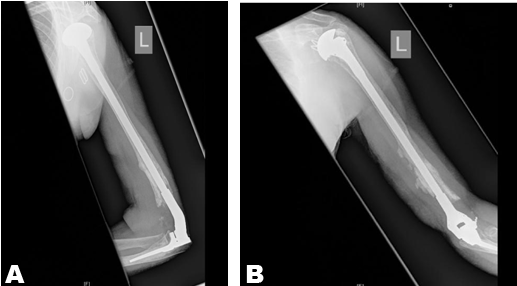
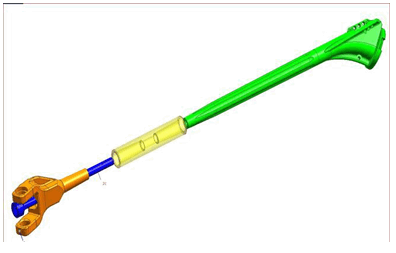
The linked elbow implant used in this study can compensate for variable degrees of bone loss, although the survival of the implant depends on many factors. According to Shah et al, these would include the overall alignment of the implant, surgical skills, as well as the quality of the patients’ bone [7][8]. Another major factor is the humeral stem length, an increase from 3.5–7 cm has increased the survival by decreasing humeral loosening Trail el al. [8][9].
The standard method of treatment for the proximal humerus is again to salvage the limb, in that reconstruction following excision of tumors of the proximal humerus gives better function and is more cost effective when compared to amputation [3][10] Allograft, arthrodesis and endoprosthetic replacement of proximal humerus (EPRPH) have all been used [10].
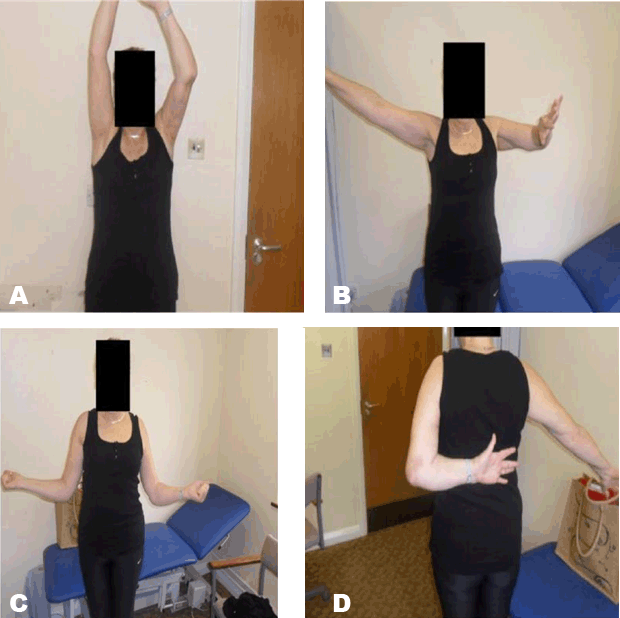
When comparing the shoulder hemiarthroplasty carried out in our patient with the formal EPRPH, it is of note that the level of bone resection was intra-articular and, as such, neither the rotator cuff nor axillary nerve was compromised. This undoubtedly resulted in improved movement, particularly abduction.
Periprosthetic fracture is the major implant related complication with ipsilateral shoulder and elbow replacements [4][11].
Many studies have tried to find a solution for this problem. One approach suggested leaving a gap of more than 6 cm between the tips of the prosthesis, [4] another one suggested minimizing this gap [11]. One more study suggested filling the space between the implants with cement [4]. Plausinis et al. studied the mechanics behind these approaches and showed that the gap between the tips of implants whether it was 5-30-60 mm did not affect the stress concentration at the tips of implants. In addition, they also showed that filling the space between the implants with cement did not significantly affect fracture rate, as only 3% of the bending forces were transmitted across the cement [12].
Stress shielding is another complication of arthroplasty that can lead to periprosthetic fractures[11][13]. The incidence of stress shielding increase with increasing the stiffness of the implant. In our case, although there is an increased risk of developing stress shielding, current radiological evaluation does not show any evidence of progressive cortical thinning or impending fractures.
|
-
Athwal GS, Chin PY, Adams RA, Morrey BF. Coonrad-Morrey total elbow arthroplasty for tumours of the distal humerus and elbow. J Bone Joint Surg Br 2005 Oct;87(10):1369–74.
[CrossRef]
[Pubmed]

-
Bashore CJ, Temple HT. Management of metastatic lesions of the humerus. Orthop Clin North Am 2000 Oct;31(4):597–609.
[CrossRef]
[Pubmed]

-
Chalidis B, Papadopoulos P, Sachinis N, Dimitriou C. One-stage shoulder and elbow arthroplasty after ipsilateral fractures of the proximal and distal humerus. J Orthop Trauma 2008 Apr;22(4):282–5.
[CrossRef]
[Pubmed]

-
Clain A. Secondary malignant disease of bone. Br J Cancer 1965 Mar;19:15–29.
[CrossRef]
[Pubmed]

-
Fuhrmann RA, Roth A, Venbrocks RA. Salvage of the upper extremity in cases of tumorous destruction of the proximal humerus. J Cancer Res Clin Oncol 2000 Jun;126(6):337–44.
[CrossRef]
[Pubmed]

-
Gill DR, Cofield RH, Morrey BF. Ipsilateral total shoulder and elbow arthroplasties in patients who have rheumatoid arthritis. J Bone Joint Surg Am 1999 Aug;81(8):1128–37.
[CrossRef]
[Pubmed]

-
Grimer RJ, Carter SR, Pynsent PB. The cost-effectiveness of limb salvage for bone tumours. J Bone Joint Surg Br 1997 Jul;79(4):558–61.
[CrossRef]
[Pubmed]

-
Harrington KD. Orthopaedic management of extremity and pelvic lesions. Clin Orthop Relat Res 1995 Mar;(312):136–47.
[Pubmed]

-
Inglis AE, Inglis AE Jr. Ipsilateral total shoulder arthroplasty and total elbow replacement arthroplasty: A caveat. J Arthroplasty 2000 Jan;15(1):123–5.
[CrossRef]
[Pubmed]

-
Kumar D, Grimer RJ, Abudu A, Carter SR, Tillman RM. Endoprosthetic replacement of the proximal humerus: Long-term results. J Bone Joint Surg Br 2003 Jul;85(5):717–22.
[Pubmed]

-
Lancaster JM, Koman LA, Gristina AG, et al. Pathologic fractures of the humerus. South Med J 1988 Jan;81(1):52–5.
[Pubmed]

-
Mansat P, Bonnevialle N. Fractures of the distal humerus: Total elbow arhroplasty. In: Stanley D, Trail I, editors. Operative Elbow Surgery. 1ed. London: Churchil livingstone; 2012.

-
McConkey MO, Baslaim AM, Regan WD. Case reports: Ipsilateral shoulder and elbow arthroplasty using custom interlocking prostheses. Clin Orthop Relat Res 2008 Oct;466(10):2548–51.
[CrossRef]
[Pubmed]

-
Nagels J, Stokdijk M, Rozing PM. Stress shielding and bone resorption in shoulder arthroplasty. J Shoulder Elbow Surg 2003 Jan–Feb;12(1):35–9.
[CrossRef]
[Pubmed]

-
Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin 1997 Jan–Feb;47(1):5–27.
[CrossRef]
[Pubmed]

-
Plausinis D, Greaves C, Regan WD, Oxland TR. Ipsilateral shoulder and elbow replacements: On the risk of periprosthetic fracture. Clin Biomech (Bristol, Avon) 2005 Dec;20(10):1055–63.
[CrossRef]
[Pubmed]

-
Shah BM, Trail IA, Nuttall D, Stanley JK. The effect of epidemiologic and intraoperative factors on survival of the standard souter-strathclyde total elbow arthroplasty. J Arthroplasty 2000 Dec;15(8):994–8.
[CrossRef]
[Pubmed]

-
Trail I. Total elbow arthroplasty. In: Stanley D, Trail I, editors. Operative Elbow Surgery. 1ed. London: Churchil livingstone; 2012.

-
Trail LA, Nuttall D, Stanley JK. Comparison of survivorship between standard and long-stem souter-strathclyde total elbow arthroplasty. J Shoulder Elbow Surg 2002 Jul–Aug;11(4):373–6.
[CrossRef]
[Pubmed]

|